[Solved] How many moles NH4Cl must be added to NH3 to 9to5Science
Ammonium chloride is an inorganic chemical compound with the formula NH4Cl and a white crystalline salt that is highly soluble in water. Solutions of ammonium chloride are mildly acidic. In its naturally occurring mineralogic form, it is known as sal ammoniac.
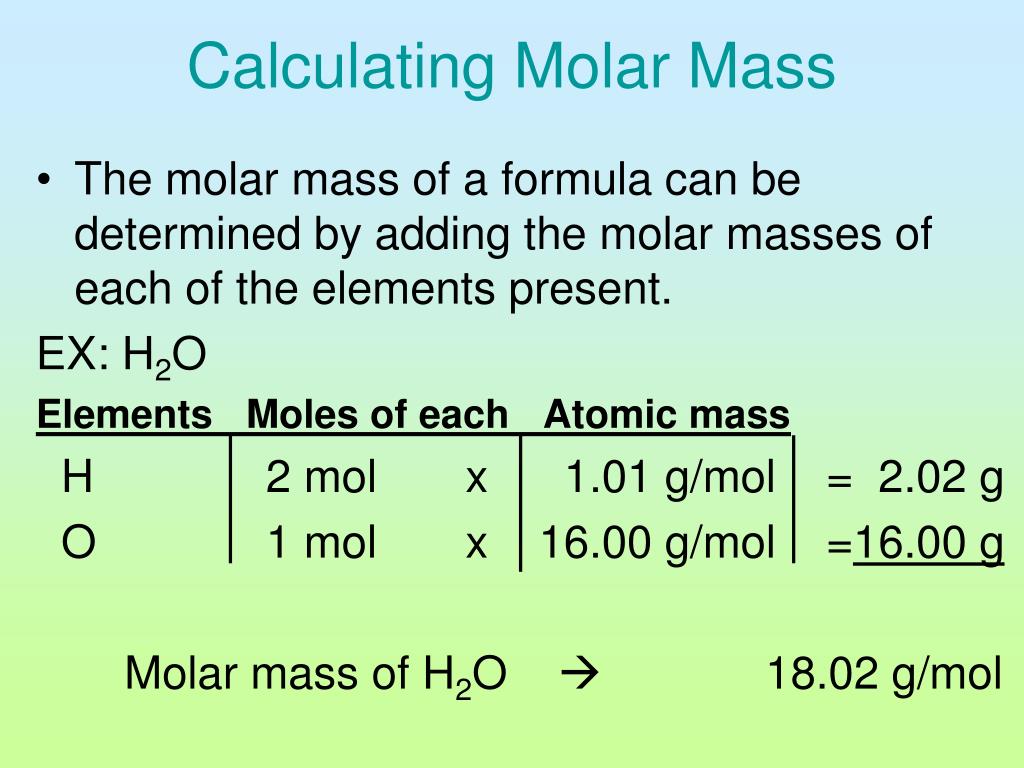
How To Calculate Molar Mass Of A Compound My XXX Hot Girl
The molar mass and molecular weight of NH 4 Cl + is 53.491. Convert NH4Cl {+} Moles to Grams Moles mol Convert Convert NH4Cl {+} Grams to Moles Weight g Convert Composition of NH 4 Cl + Element - Mass Percent Chlorine 35.453g Chlorine 35.453g Nitrogen 14.0067g Nitrogen 14.0067g Hydrogen 4.0318g Hydrogen 4.0318g 🛠️ Calculate Molar Mass Instructions

Stepwise chlorination of a 20 mM NH4Cl solution with different molar... Download Scientific
NH4Cl Molar/atomic mass: 53.49 Sublimation point (°C): 337.6 Solubility (g/100 g of solvent): 1,4-dioxane: 0.000135 (25°C) 1,4-dioxane: 0.000182 (30°C) 1,4-dioxane: 0.000255 (35°C) 1,4-dioxane: 0.000442 (45°C) 1,4-dioxane: 0.00107 (55. Standard molar enthalpy.
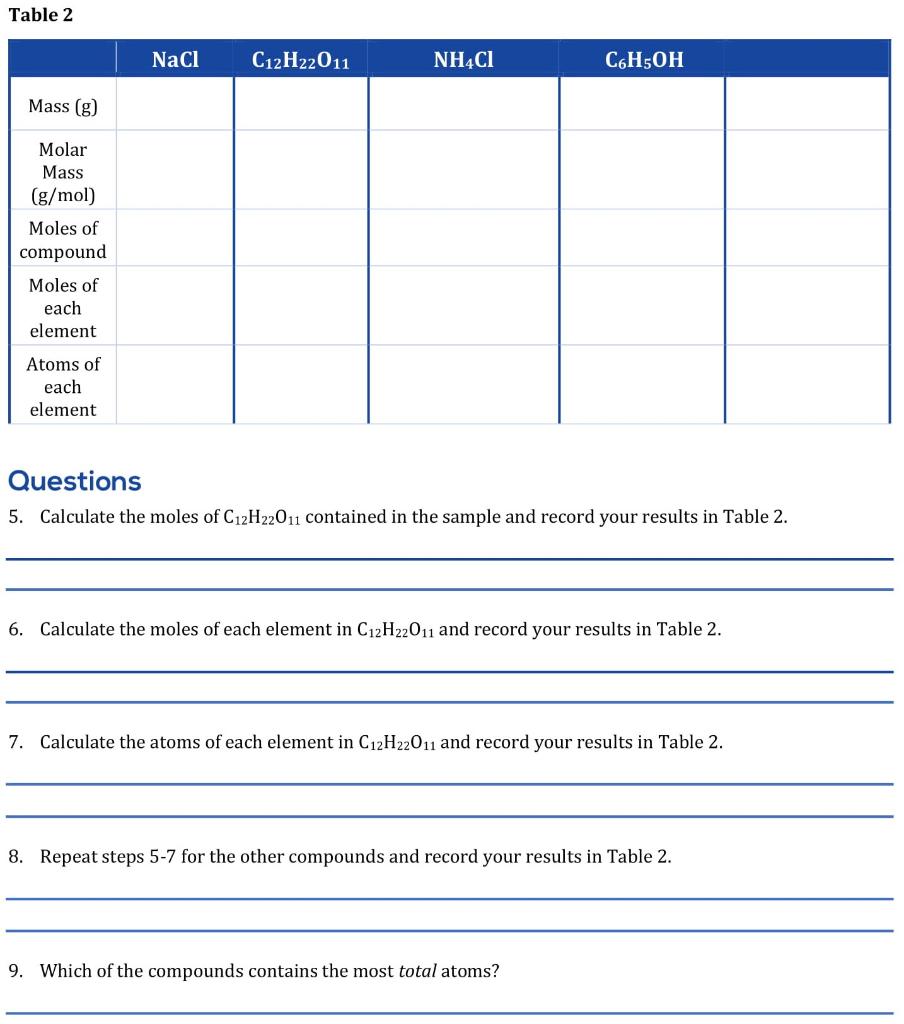
Table 2 NaCl C12H22011 NH4Cl C6H5OH Mass (g) Molar
How many grams NH4Cl in 1 mol? The answer is 53.49146. We assume you are converting between grams NH4Cl and mole . You can view more details on each measurement unit: molecular weight of NH4Cl or mol This compound is also known as Ammonium Chloride .

Molar Mass of NH4Cl (Ammonium chloride) YouTube
Ammonium Chloride NH4Cl Molar Mass, Molecular Weight. ENDMEMO. Home » Chem » Name Formula Search; Ammonium Chloride. Name: Ammonium Chloride. Formula: NH4Cl. Molar Mass: 53.4915. NH4Cl Molar Mass Converter. Weight: Mole: Example Reactions: • NH3 + HCl = NH4Cl • NH4Cl + NaOH = NH3 + H2O + NaCl • NH4Cl + KOH = NH3 + H2O + KCl.
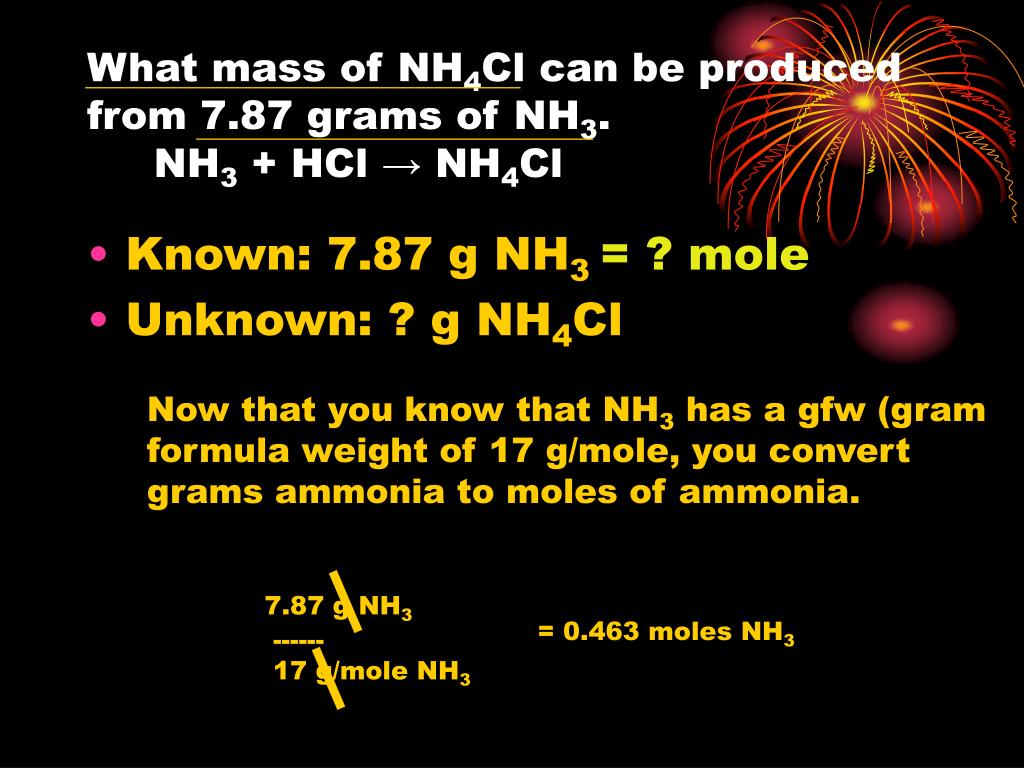
PPT Procedure Checklist PowerPoint Presentation, free download ID4501650
Molar mass of NH4Cl (Ammonium chloride) is 53.4915 g/mol Get control of 2022! Track your food intake, exercise, sleep and meditation for free. Convert between NH4Cl weight and moles Elemental composition of NH4Cl Chemical structure Appearance Ammonium chloride appears as a white crystalline solid. Sample reactions for NH4Cl Related compounds
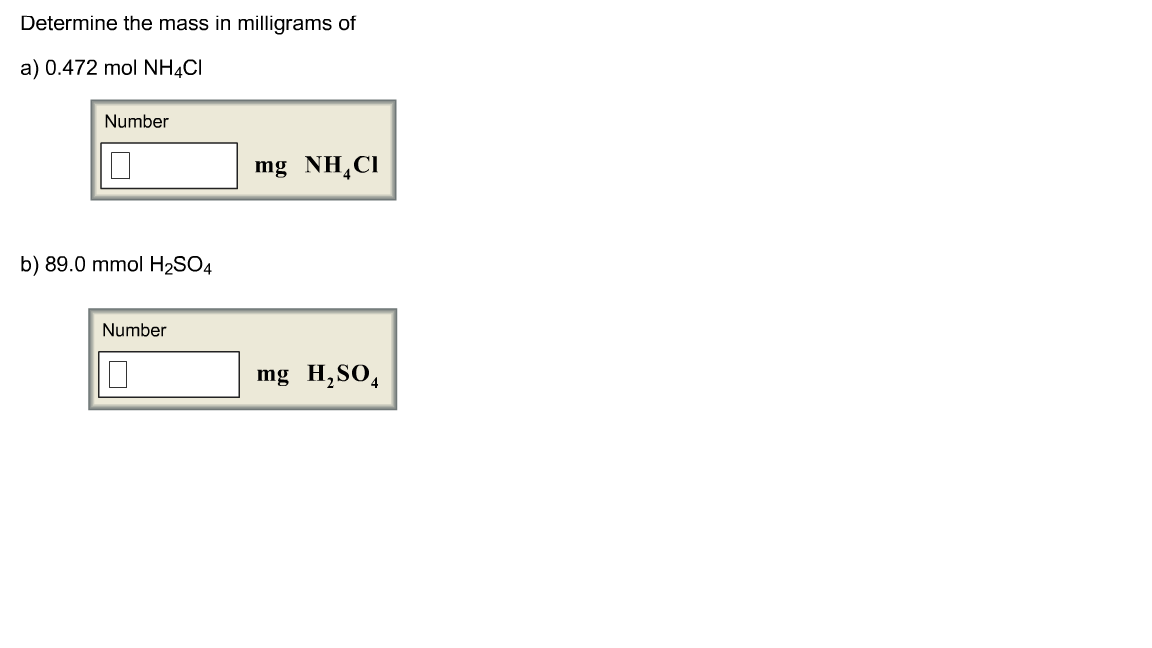
Solved Determine the mass in milligrams of 0.472 mol NH4Cl
Calculate the molar mass of the following: NH4Cl (Ammonium Chloride).more

Solved Mole Conversions What is the molar mass of 1. NH4CL
Quantity Value Units Method Reference Comment; Δ f H° solid-314.55: kJ/mol: Review: Chase, 1998: Data last reviewed in September, 1965: Quantity Value Units Method Reference
Molar Mass / Molecular Weight of NH4Cl Ammonium chloride YouTube
Formula The molar mass of NH4Cl (ammonium chloride) is: 53.489 grams/mol. See also our theoretical yield calculator for chemical reactions (probably your next stop to finish the problem set). Tool Overview: Molar Mass of ammonium chloride (NH4Cl) Solving for the atomic mass of ammonium chloride (NH4Cl)

How to find the molecular mass of NH4Cl (Ammonium Chloride) YouTube
Molar mass of NH4Cl (aq) is 53.4915 g/mol Get control of 2022! Track your food intake, exercise, sleep and meditation for free. Convert between NH4Cl (aq) weight and moles Elemental composition of NH4Cl (aq) Formula in Hill system is ClH4N Computing molar mass (molar weight)

Nh4cl Molar Mass
Molar mass (molar weight) is the mass of one mole of a substance and is expressed in g/mol. Mole is a standard scientific unit for measuring large quantities of very small entities such as atoms and molecules. One mole contains exactly 6.022 ×10 23 particles (Avogadro's number)

10g of NH4Cl (molar mass 53.5 gm mol^1 ) when dissolved in 1000 gm water lowered the freezing
Watch on NH4Cl (Ammonium chloride) Molar Mass Calculation If you have a periodic table with you, then you can easily calculate the molar mass of NH4Cl (Ammonium chloride). Because the molar mass of any molecule (or compound) can be calculated by simply adding the molar masses of individual atoms.

The avergae molar mass of the vapour above solid NH4Cl is nearly 26.5 g mole^1 . Find the
NH4Cl molecular weight Molar mass of NH4Cl = 53.49146 g/mol This compound is also known as Ammonium Chloride. Convert grams NH4Cl to moles or moles NH4Cl to grams Molecular weight calculation: 14.0067 + 1.00794*4 + 35.453 Percent composition by element Element:Chlorine Symbol: Cl Atomic Mass: 35.453 # of Atoms: 1 Mass Percent: 66.278%

Ammonium chloride NH4CL mole to weight YouTube
Explanation of how to find the molar mass of NH4Cl: Ammonium chloride.A few things to consider when finding the molar mass for NH4Cl:- make sure you have the.
Calculate the mass of 6.002 ×10^23 molecules of NH4cl
Ammonium Chloride | NH4Cl or ClH4N | CID 25517 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. JavaScript is required. Please enable Javascript in order to use PubChem website.

Ammonium chloride NH₄Cl Molecular Geometry Hybridization Molecular Weight Molecular
The chemical formula of the ammonium chloride is NH4Cl and it has a molar mass of 53.490 g mol-1. Moreover, this molecule is formed by two potassium cations K1+ and 1 dichromate anion Cr2O72-. Furthermore, it is present in both the forms that are the anhydrous and the three hydrate. Its chemical structure is shown below in the form of an image.